Answers to Questions Received at the ifia JAPAN 2025 Exhibition
Introduction
My name is Murase from the Food Business Division.
Our company exhibited at "ifia JAPAN 2025 – The 30th International Food Ingredients & Additives Exhibition and Conference", held at Tokyo Big Sight from Wednesday, May 21 to Friday, May 23, 2025, following our participation last year.
During the exhibition, we had the valuable opportunity to engage directly with many visitors who showed strong interest in our technologies.
At our booth, we introduced our latest initiatives to these attendees. One of the main highlights was the launch of our new solution, “digzyme Custom Enzyme Lab”
(For more details, please refer to our press release:https://prtimes.jp/main/html/rd/p/000000018.000050097.html)


The launch received an overwhelmingly positive response, far exceeding our expectations. Our booth was filled with lively discussions throughout the exhibition, as we received numerous specific questions and inquiries from many visitors each day.
In this special edition of our tech blog, commemorating the launch of “digzyme Custom Enzyme Lab”, we’ve selected some of the most frequently asked questions from the exhibition and provided detailed answers in a Q&A format.
This post is not only for those interested in our new solution, but also for anyone curious about enzyme-based development who may be wondering where to start.
We hope you’ll find useful insights—please read on to the end!
Q: For what types of product development can “digzyme Custom Enzyme Lab” be applied?
A:“digzyme Custom Enzyme Lab” is a flexible solution that can be applied to a wide range of development themes—from specific goals such as improving the efficiency of existing enzyme-based manufacturing processes to broader, more exploratory themes like developing novel food ingredients using enzymes.
By repeatedly exchanging purified enzyme samples and receiving feedback from your in-house evaluations, the development direction can be adjusted flexibly at each stage.
Q: What kind of information is provided with the purified enzyme samples?
A:We perform preliminary testing to confirm enzyme activity and provide a profile including optimal temperature, optimal pH, thermal stability, and pH stability. These data are provided alongside the purified enzyme samples.
Verification in your specific application or evaluation system can be conducted by your team.
Q:What is the quantity of purified enzyme included in the sample?
A:The quantity depends on the development theme and is determined through consultation. As a general guideline, samples are typically provided in volumes of several milliliters of enzyme solution, equivalent to several milligrams of protein.
Q:How do you define or set the initial development timeline?
A:Following a prior evaluation of the requested development theme, we assess the feasibility and propose an initial development timeline.
In most cases, the initial phase—covering in silico enzyme design through to the first delivery of a purified enzyme sample—is completed within 2 to 6 months.
Q:Is non-GMO enzyme development an option?
A:Yes, it is possible. For more details, please refer to the “digzyme Express” introduction page:https://www.digzyme.com/cms/wp-content/uploads/digzyme_Express_ol.pdf
Q:Is “digzyme Custom Enzyme Lab” a solution exclusively for the food industry?
A:“digzyme Custom Enzyme Lab” is a versatile solution available for use not only in the food industry but also in other sectors, including the chemical industry.
Q:If a suitable enzyme is found among the provided purified enzyme samples, what happens next?
A:Enzymes developed via “digzyme Custom Enzyme Lab” can smoothly transition into manufacturing development. digzyme provides comprehensive support throughout the entire process, including manufacturing technology development and regulatory approvals, accompanying you until your project is fully commercialized.
Q:How is intellectual property handled for the developed enzyme library?
A:If you find a promising enzyme among those developed via “digzyme Custom Enzyme Lab” and decide to pursue its commercialization, we are prepared to accommodate your needs flexibly.
This concludes our responses regarding the services provided through “digzyme Custom Enzyme Lab”.
Please feel free to contact us anytime, as we remain flexible and ready to accommodate your specific needs during the actual development process.
Thank you very much for reading through this Q&A.
If you have any questions or require further clarification, please do not hesitate to reach out to us via the contact form below.
[▼ Contact Form]
https://www.digzyme.com/contact/
Prediction of Enzyme Thermal Stability by Computational Methods
Koichi Tamura
(Research and Development Department)
Introduction
Enzymes are biopolymers (mainly proteins) that catalyze a wide variety of chemical reactions. Due to their lower environmental impact and high selectivity compared to metal catalysts, enzymes are widely used in industrial, food, and other applications. In general, the rate of chemical reactions depends on temperature; as the temperature increases, the reaction rate also rises. This is because heating provides the energy needed to overcome the reaction's activation barrier. For example, the Haber-Bosch process, which synthesizes ammonia from nitrogen and hydrogen using inorganic iron-based catalysts, operates at temperatures approaching 500°C.
In contrast, enzymes, being biopolymers, cannot withstand such extreme conditions and unfold (denature) at a certain temperature. This temperature is referred to as the melting temperature (Tm). Tm is a critical indicator for assessing an enzyme's thermal stability, and predicting and improving Tm is a key challenge in protein engineering. At digzyme, we have developed a computational method to predict scores correlated with enzyme Tm, enabling the selection of enzymes that function at high temperatures and the improvement of enzyme performance to meet a broader range of needs.
In this blog, we evaluate the performance of our developed method using two datasets of experimentally derived protein thermal stability data.
Methodology
In the context of enzyme discovery and modification, target enzymes for thermal stability prediction often originate from a wide variety of species and protein families. Therefore, prediction models require versatility, independent of these two factors. To meet this requirement, various methods have been proposed, which can be broadly categorized into the following two approaches:
1. Data-Driven Approaches
This approach, often referred to as machine learning, involves constructing predictive models for protein thermal stability based on large datasets derived from experimental results. The process of building these models is called training. The duration of training depends on the amount of data and the complexity of the model, ranging from the time it takes to enjoy a cup of coffee to an entire week of computation using cutting-edge GPGPU (General-Purpose Graphics Processing Unit) systems. Once a trained model is established, the computational cost for subsequent predictions is significantly lower than during the training phase.
As suggested by the model construction process, the accuracy of machine learning models heavily depends on the quantity and quality of the training data. Limited data introduces bias into the inferred rules, and care must also be taken to ensure the training data is not skewed toward specific species or protein families. Additionally, consistent experimental conditions for measuring protein properties are desirable, although achieving this is challenging given that data in current databases originates from various research groups.
Even with attention to data quantity and bias, generalizability issues may persist, primarily due to overfitting and extrapolation challenges. Overfitting occurs when a machine learning model overly adapts to the training data, yielding excellent predictions for the training set but failing with new data. Preventing overfitting requires expert techniques, such as appropriately controlling model complexity. Extrapolation refers to the model's ability to maintain reliability for data outside the training range. For proteins, this means being able to make reliable predictions for novel amino acid sequences with low homology to the training data. This capability is critical in enzyme discovery and modification because the target sequences often show low similarity to previously studied enzymes. While extrapolation is a crucial feature, building models inherently equipped with it is difficult except for simple linear models. Thus, the best practice here is to thoroughly understand and respect the applicability domain of the constructed machine learning models.
2. Physics-Based Approaches
Physics-based models begin by constructing energy functions that describe atomic interactions. The universal natural laws governing atomic and molecular systems have been mathematically formulated for over a century. Simple examples allow manual solutions to these equations, enabling comparison with experimental values to validate their accuracy (see introductory physical chemistry textbooks for details). Since these equations include no arbitrary parameters apart from physical constants (e.g., the speed of light, Planck's constant), they offer universal applicability. However, for complex multi-atomic systems like enzymes in solvents, these equations become highly intricate, making their practical computation infeasible even with state-of-the-art computational resources.
To address this, approximations are introduced to simplify the equations, trading off precision and universality for computational feasibility. Common approximation methods include:
●Empirical Energy Functions in Molecular Mechanics: These functions simplify inherently complex atomic interactions by assuming basic functional forms and introducing parameter sets.
●Treatment of Solvents via Statistical Mechanics: Instead of explicitly modeling numerous solvent molecules around the enzyme, their effects are replaced with averaged quantities.
These approximations significantly reduce computational costs but at the expense of accuracy and universality.
digzyme Score
At digzyme, we adopt a physics-based approach to predict enzyme thermal stability. Our model takes three-dimensional structural information of enzymes as input and outputs a score—referred to as the "digzyme score." This score is designed to correlate with the enzyme's melting temperature (Tm) and typically falls near a value of 1.0. While it does not directly predict the exact Tm value, this design suffices for practical purposes, such as comparing the relative stability of multiple enzymes.
Case Study 1: Prediction of Mutant Thermal Stability
In order to computationally design and select useful enzyme mutants, it is necessary to calculate various properties of numerous candidate mutants and rank them based on their values. Among these properties, thermal stability is particularly important. Below, we compare the results of multiple groups and digzyme in a competition to predict the difference in unfolding free energy (ΔΔGu) between the wild-type and mutants of frataxin, an enzyme involved in iron-sulfur cluster regeneration.
A competition was held in 2018 to predict the difference in unfolding free energy (ΔΔGu) between the wild-type and eight mutants of frataxin[1], and the results were published in a paper in 2019[2]. Unfolding free energy (ΔGu) is defined by the equation (1), where...
ΔGu = Gu - Gf (1)
It is defined as the change in free energy associated with the unfolding of the enzyme. Here, Gu and Gf represent the free energies of the unfolded and folded states, respectively. In general, since the folded state of the enzyme is more stable, ΔGu is greater than 0. Note that the larger the ΔGu, the more difficult it is for the enzyme to unfold (i.e., the more stable it is). For both the wild-type and mutant forms, the unfolding free energy defined by equation (1) is measured, and by calculating the difference between them, the change in unfolding free energy due to the mutation can be computed as follows:
ΔΔGu = ΔGu(mutant) - ΔGu(wild-type) (2)
ΔΔGu < 0 Then, it means that the enzyme has become destabilized due to the mutation.
Figure 1 on the left shows the predicted score ("digzyme score") calculated by digzyme and the experimental values. The Pearson correlation coefficient was 0.87. This result is slightly better than the existing popular physics-based method, FoldX (Figure 1 on the right).
Another physics-based method was used by the Pal Lab group, which employed a molecular dynamics (MD)-based approach for prediction. In this method, structural sampling (1 ns) is performed using MD, and the sampled structures are clustered into folded and unfolded states. Subsequently, energy calculations are performed for each representative structure in the clusters, and the values from equation (1) are calculated. By running this calculation for both the wild type and mutant, values can be obtained that can be compared with experimental data using equation (2). However, the assumption that the protein in the folded state (MD starting structure) will undergo a structural transition to the unfolded state within 1 ns under the given calculation conditions (27°C, typical equilibrium MD) is clearly unrealistic. This is because even for fast-folding proteins, which fold/unfold relatively quickly, simulations near their Tm still take over 1,000 times longer for a structural transition to occurr[3], the moderate correlation found using this method (Figure 1 on the right) is arguably a result of chance.
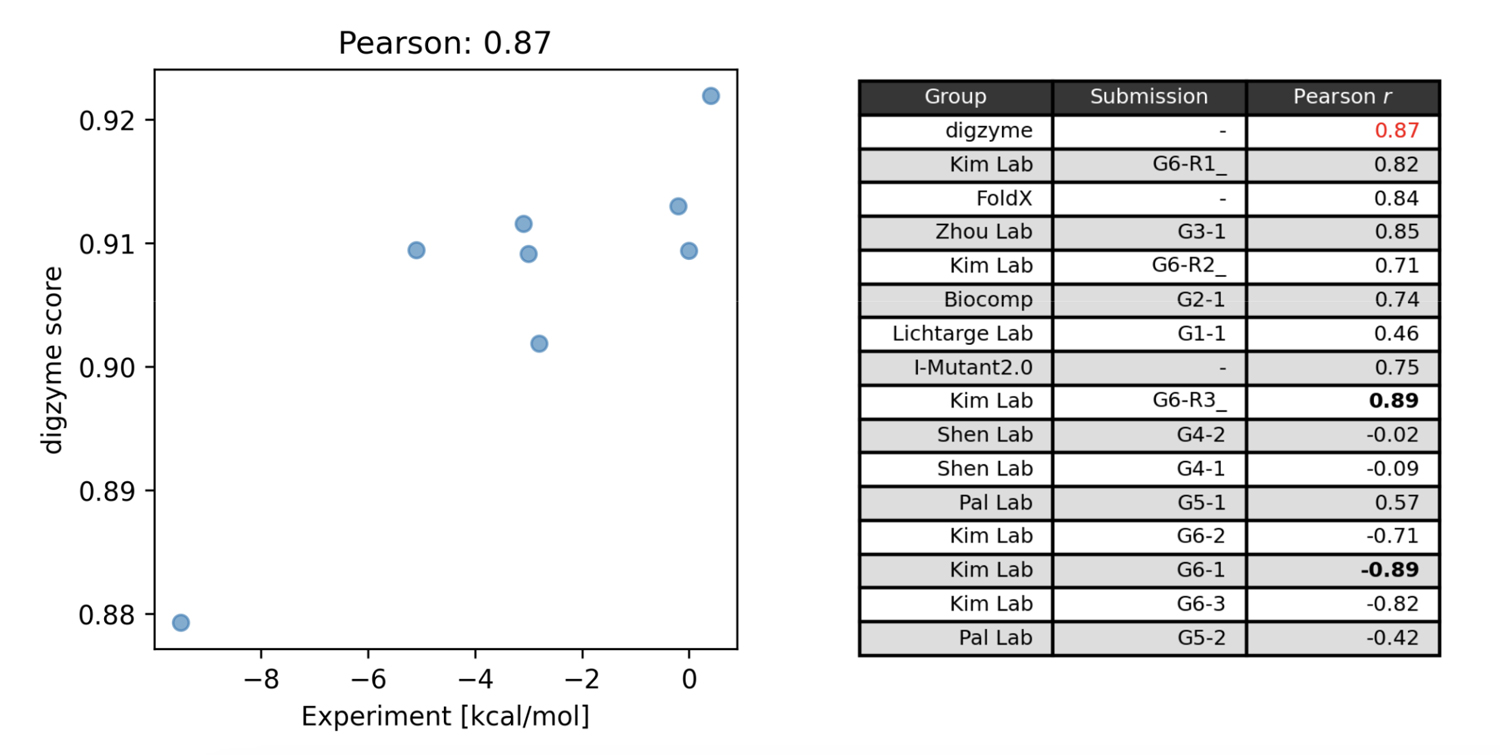
The group that proposed the model with the highest correlation in the contest was the Kim Lab group, with an absolute correlation coefficient of 0.89 (Figure 1, right). This group used a machine learning model for predictions, utilizing thermal stability data of mutants registered in the Protherm database for training [4]. In this machine learning model, both structure-based and amino acid sequence-based features are calculated, and a regression model is built using gradient boosting trees with these features. Among the structure-based features, the calculated values from the physical model FoldX are included, and it is known that these values are the most important features [4]. This fact suggests that using the digzyme score, a physical model with similar accuracy to that of FoldX, could help build even more advanced machine learning models. If there is a large amount of mutant data available for the target enzyme, it may be worth attempting to build a dedicated model.
Case 2. Prediction of Thermal Stability of Enzymes with the Same Function
In the search for useful enzymes, a large number of amino acid sequences of enzymes with the same (predicted) function are extracted from a database (forming the population), and candidate enzymes are selected by ranking them based on some criteria. Similar to mutant design, thermal stability is one of the important indicators in this process.
It is important to note that the prediction of thermal stability here is different from the prediction of the difference between wild-type and mutant enzymes as seen in Case 1. Typically, the length of the amino acid sequence is the same for both wild-type and mutant enzymes, and the sequence identity is often close to 99%. In contrast, in a population of sequences extracted from a database for a specific function, the length of the amino acid sequences varies, and the sequence identity is generally low. Predicting thermal stability and creating a ranking of sequences in such a population can often be challenging, as shown below.
The example here is a large-scale dataset of the Tm (melting temperature) of nanobodies (small fragments of antibodies) called NanoMelt, which was posted on a preprint server in September 2024[5]. This dataset includes both existing data and newly measured data by the authors (640 data points), with experimental conditions such as protein concentration, pH, and buffer standardized. For this dataset, the digzyme score was calculated for each enzyme using the same physical model as in Case 1, and the correlation with experimental values was examined.
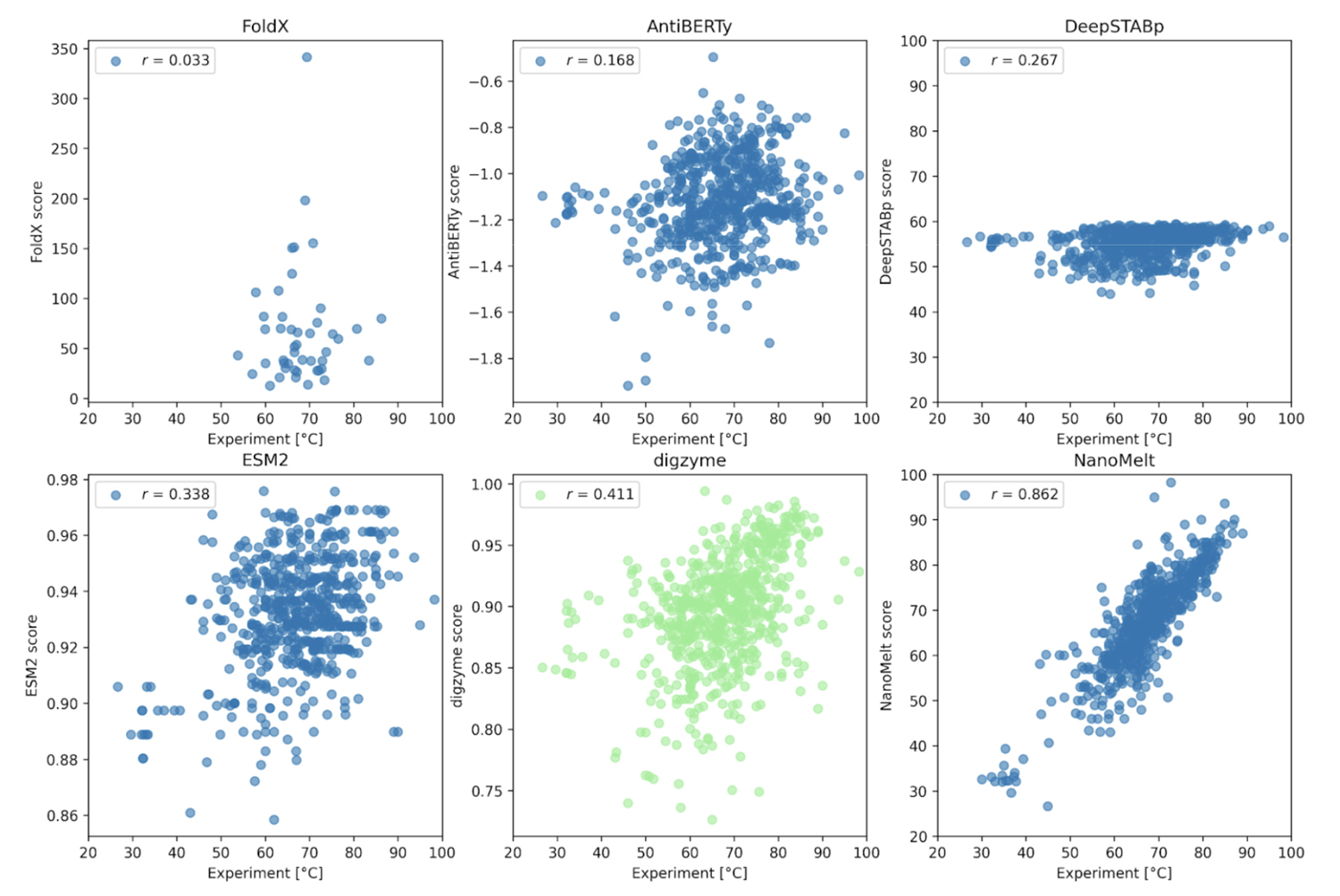
As shown in Figure 2, except for the results where the NanoMelt dataset itself was used for training (Figure 2, lower right), there was no or only weak correlation between the experimental values and the prediction results. First, FoldX, a physical model that achieved a high correlation coefficient in Case 1, failed to provide a correlated prediction result here. In contrast, digzyme successfully predicted a weakly correlated result (r = 0.411, Figure 2, lower center). Even when limiting the data to the 46 amino acid sequences used in FoldX predictions, a weak correlation was observed (r = 0.273), which signifies a remarkable advancement in expanding the applicability of existing physical models. AntiBERTy and ESM-2 are protein language models, and the scores in Figure 2 represent the (pseudo) log-likelihood of the amino acid sequences [5]. This is an indicator of the sequence's probability or plausiblity, and some correlation with thermal stability was expected. However, the actual correlation was weak (correlation coefficients of 0.168 and 0.338, respectively). Therefore, as the authors suggest, if these language models are used for thermal stability prediction, it would be better to build a model specialized for the task through further training [5]. On the other hand, the low correlation of DeepSTABp, a dedicated regression model for predicting Tm (r =0.267, Figure 2, upper right), requires careful consideration. According to the original paper [6], the Pearson correlation coefficient for DeepSTABp's test data was 0.90, yielding significant results. Despite this, the low correlation with the NanoMelt dataset suggests that this model may have overfitted and has limitations in generalization ability (i.e., it is powerless against "unfamiliar data"). In contrast, the physical model adopted by digzyme is based on general physical principles, which have no inherent limits to the range of amino acid sequences they can be applied to. In fact, since digzyme's model gives a higher correlation than DeepSTABp, it can be said that the superiority of the physical model is demonstrated to some extent.
Now, the NanoMelt model, which shows a strong correlation (r = 0.862, Figure 2, lower right), raises concerns about potential overfitting. Unfortunately, since NanoMelt is a nanobody-specific model, it is difficult to re-evaluate its performance on other datasets containing arbitrary protein sequences. Therefore, the authors selected six new sequences from the camel nanobody sequence database and experimentally measured Tm to re-evaluate the model. The criteria for selecting the sequences were as follows:
1.The dissimilarity with the most similar sequence in the NanoMelt dataset is at least 30%.
2.The AbNatiV VHH-nativeness score (a measure of sequence likelihood) is 0.85 or higher.
3.The predicted Tm in NanoMelt is either low (Tm < 61°C) or high (Tm > 73°C).
Of these six sequences, the three predicted to have low Tm did not express, but the three predicted to have high Tm did express, and successful Tm measurements were made. The error in the predicted results was approximately 1°C, demonstrating the high predictive performance of NanoMelt [5]. While this result does not definitively determine the presence of overfitting, it provides a clue to understanding the applicable range of the NanoMelt model.
Conclusion
In the context of enzyme discovery and modification, calculating the thermal stability of enzymes and creating an accurate ranking is crucial to reducing subsequent experimental processes. At digzyme, we tackle this challenge by applying a general-purpose physical model. In this blog, we evaluated our developed model by calculating prediction scores for two datasets. As shown in Case 1, we demonstrated that our model achieves practical accuracy in predicting the thermal stability of variants. However, as shown in Case 2, while we showed the superiority of our model over existing physical and machine learning models in comparing arbitrary amino acid sequences, the correlation with experimental values was weak, indicating room for further improvement.
Recently, there have been increasing successful cases of enzyme design using AI, with expectations for realizing novel folds (structures) and functions that were overlooked during the evolutionary process. However, the novelty of these designs makes predictions of physical properties, such as thermal stability, by machine learning challenging. Therefore, the importance of general-purpose physical models, such as those introduced in this blog, is expected to grow even more. At digzyme, we plan to continue actively advancing research and development to build more accurate and reliable models.
References
[1] CAGI5 Frataxin [Link]
[2] Savojardo et al. Hum. Mutat., 2019, 40(9), 1392 [Link]
[3] Lindorff-Larsen et al. Science, 2011, 334, 517 [Link]
[4] Berliner et al. PLoS ONE, 2014, 9(9), e107353 [Link]
[5] Ramon et al. bioRxiv, 2024 [Link]
[6] Jung et al. Int. J. Mol. Sci., 2023, 24(8), 7444 [Link]
Search and visualization of polysaccharides containing target monosaccharides from glycan databases.
Summary
My name is Ryu Takayanagi, a second-year master's student at the University of Tokyo, currently interning at digzyme. At university, I have been conducting research related to protein phosphorylation and protein tertiary structures.
In this tech blog, I would like to introduce GlycoSearcher, a new tool we have developed as part of our R&D activities for comprehensive search and visualization of polysaccharides containing target monosaccharides.
In recent years, research and industrial utilization of polysaccharides, such as starch and dietary fiber, have become increasingly active. There is a growing demand for the development of new saccharides, and polysaccharides, in particular, are gaining attention for their high structural diversity. To meet this need, we have developed GlycoSearcher, a tool for comprehensive search of various polysaccharides.
Description formats and databases of polysaccharides
The glyco-compounds we are focusing on have already been reported in numbers exceeding hundreds of thousands and have been databased. To selectively identify polysaccharides that fit specific purposes and apply them in fields such as synthesis pathway exploration and enzyme development, a description format that facilitates computational processing and a comprehensive database are essential.
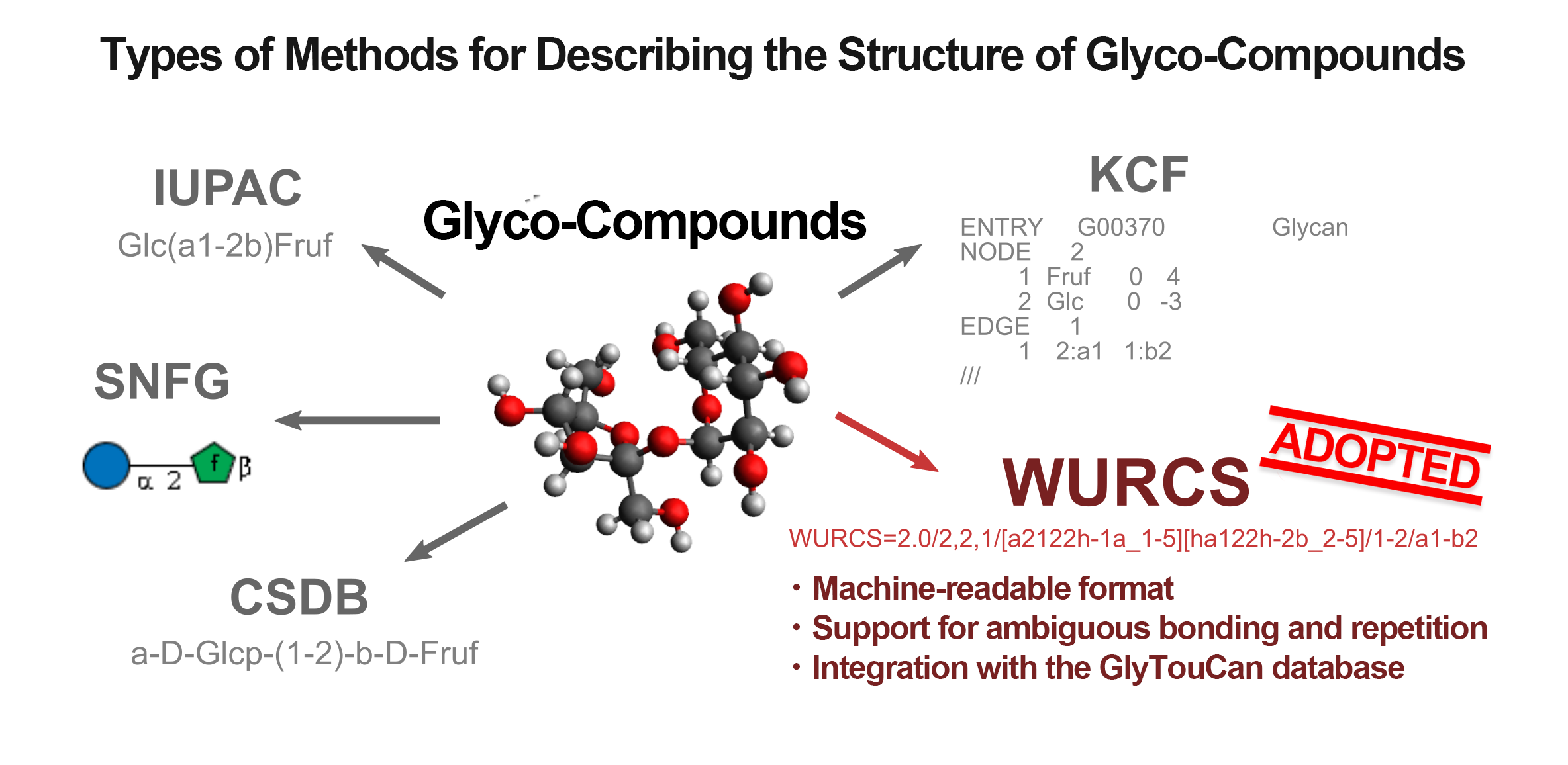
Various methods are known for describing the structure of glyco-compounds (Figure 1). Formats like SNFG and KCF excel in visualization but are not well-suited for advanced computational processing, such as structural information extraction and comparison. On the other hand, the IUPAC format offers a concise structural representation that is readable by both humans and machines, but it struggles with complex and ambiguous expressions, such as repeating units[1]. Therefore, GlycoSearcher employs the WURCS format, which is well-suited for computational processing and can represent repeating units, along with the GlyTouCan database[2], which collects glyco-compound information in the WURCS format.
(Figure 1)
Search Using GlycoSearcher
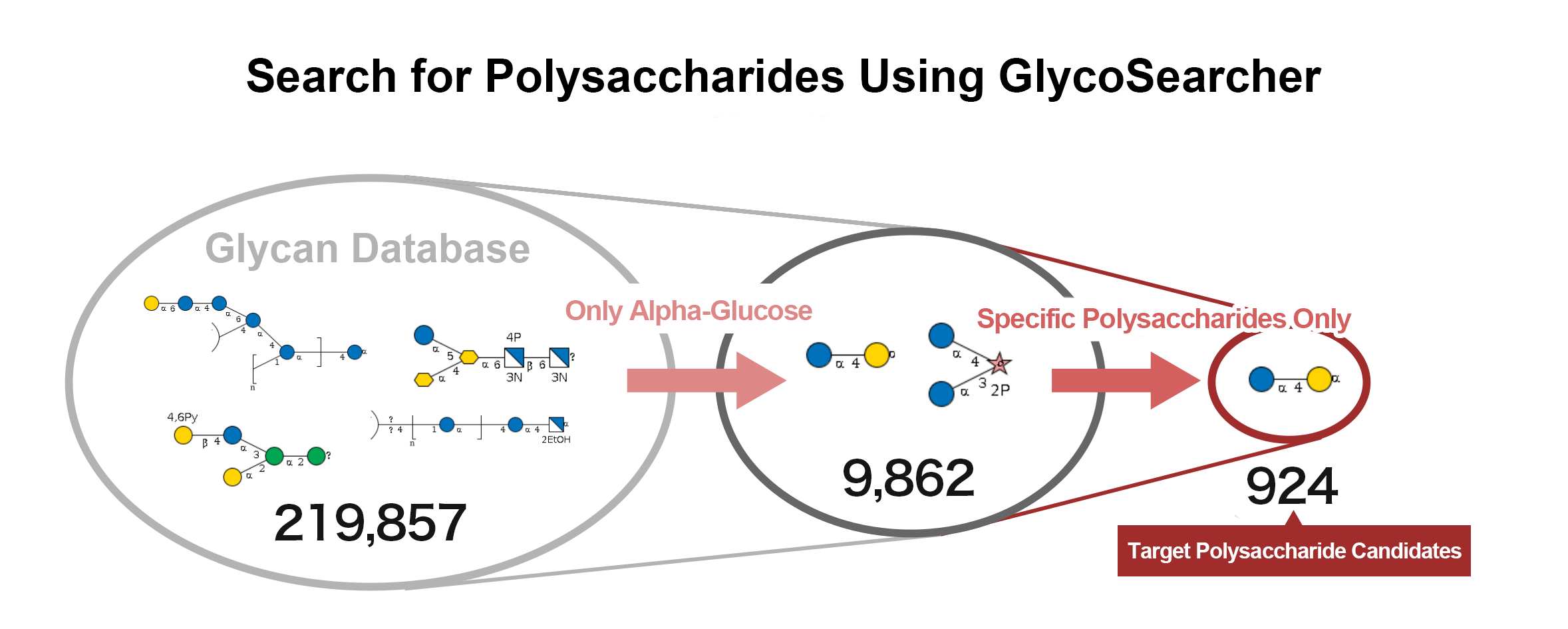
With GlycoSearcher, it is possible to extract polysaccharides that match specific criteria from a vast number of candidates. For example, you can search for polysaccharides containing particular monosaccharide units such as glucose or galactose. Additionally, it includes a filtering function that allows you to limit the monosaccharide units that make up the polysaccharides. This enables you to list polysaccharides that can be synthesized using a specific monosaccharide as a starting material and other selected sugars.
The results of a search for polysaccharides containing α-glucose are shown below (Figure 2). Out of 219,857 glycan structures, 9,862 polysaccharides containing α-glucose were identified. Further narrowing down the search to polysaccharides consisting only of glucose, galactose, and fructose reduced the number of candidates to 924.
(Figure 2)
Visualization and feature extraction of polysaccharide structures
The obtained search results can be effectively visualized and utilized for subsequent applications (Figure 3). By reconstructing polysaccharides in WURCS format as graphs, it is possible to rapidly visualize thousands of search results within minutes. Additionally, for structures with ambiguous repeating units, repeating only a specific number of times allows not only the visualization of the actual structure but also facilitates further computational processing, such as structural comparisons that are challenging when ambiguous.
Since the polysaccharides in the search results are represented as graphs, feature extraction for polysaccharide structures is also possible. For example, computations can determine whether the obtained polysaccharide structures have glucose units at their termini or include specific structures (motifs). Furthermore, by integrating the hit polysaccharides with various databases such as PubChem[3], it is possible to obtain information on their common names and related enzyme information, thus providing insights into reactions involving the polysaccharides.
(Figure 3)
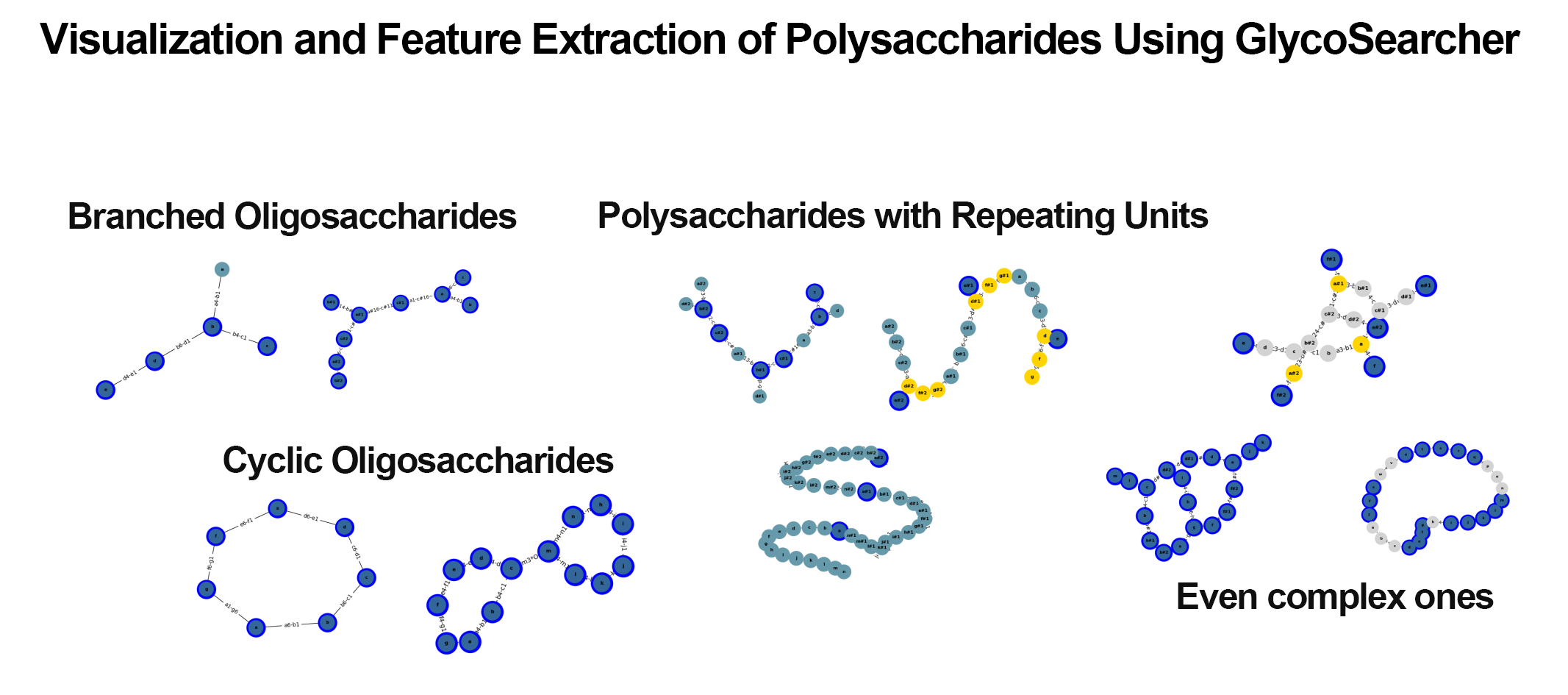
Conclusion
The GlycoSearcher we developed allows for comprehensive searching of target polysaccharides from the database and facilitates further computational processing. Additionally, by extracting information from the identified target polysaccharide candidates and obtaining enzyme information predicted to be involved in their synthesis, we have established a system that links to subsequent enzyme design workflows.
Acknowledgments
The development of GlycoSearcher, including acquiring knowledge about glyco-compounds, was greatly supported by Mr. Isozaki from the Business Development Department. I would like to take this opportunity to express my gratitude.
References
[1] Hosoda, M., & Kinoshita, S. (2021). "Introduction to Glycan-related Informatics." JSBi Bioinformatics Review, 2(1), 87-95.
[2] GlyTouCan. Retrieved from https://glytoucan.org/
[3] PubChem. Retrieved from https://pubchem.ncbi.nlm.nih.gov/
List of answers to the questions we received at our booth at ifia JAPAN 2024 venue.
Our company exhibited at "ifia JAPAN 2024 - The 29th International Food Ingredients/Additives Exhibition & Conference" (organized by Food Chemical News Co., Ltd.), held at Tokyo Big Sight from May 22nd (Wednesday) to 24th (Friday), 2024.
We would like to express our sincere gratitude to everyone who visited our exhibition booth.
In this article, we will introduce and answer the questions we received from all of you during the exhibition period, focusing on the ones that were particularly frequent. Please stay tuned until the end.
Q: What does your company do?
A: In response to our clients' needs, we conduct new enzyme exploration and enzyme modification. By employing our unique bioinformatics technology, which differs from conventional methods, we facilitate rapid enzyme development. We believe that this innovation accelerator can benefit both enzyme manufacturers and food manufacturers.
Q: Do you have any specific examples?
A: In chemical applications, we have successfully explored new enzymes needed by users and achieved significant improvements in enzyme activity. In food applications, we are currently addressing specific themes requested by multiple clients and actively working on them.
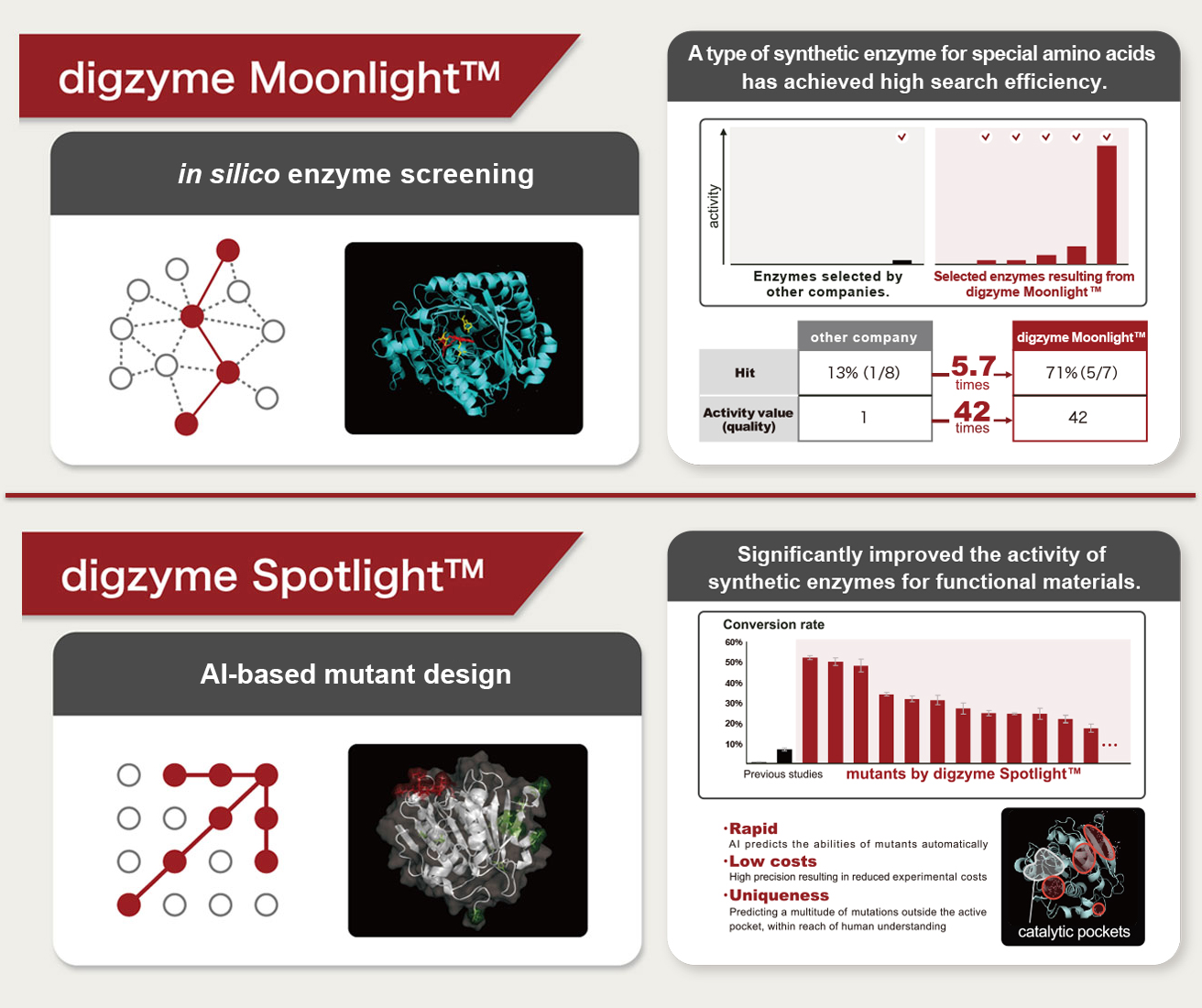
Q: What properties of enzymes can be modified in the digzyme Spotlight (enzyme modification program)?
A: Potential modifications include enhancing activity, improving heat resistance, and altering optimal pH. Modifications to substrate specificity are addressed using the digzyme Moonlight (enzyme exploration program) as needed.
Q: What is the development process like?
A: Depending on the client's situation, we set the start and end goals, but the main process typically follows these steps:
- Development Consultation: We listen to the client's challenges and select the target enzymes.
- Enzyme Design: Using supercomputers, we design the target enzymes.
- Enzyme Library Provision: Enzymes designed on the computer are produced at the lab scale using microorganisms, and the suitability of the enzymes for the intended purpose is verified and confirmed.
- Enzyme Production Provision: We scale up production from the lab to the plant, ensuring stable enzyme supply as a product.
That concludes the Q&A for this article. Thank you very much for reading until the end. If you have any further questions or inquiries, please contact us using the following contact form.
[Contact Form] https://www.digzyme.com/contact/
Compare the predictive accuracy of the machine learning model Spotlight™ for enzyme variants’ activity with prior research.
Summary
Our company offers a service called Spotlight that uses a machine learning model to suggest mutants that improve properties such as enzyme activity and thermostability. We input target enzyme sequences into a pre-trained model using various enzymes to predict mutants with improved activity and thermostability for that enzyme. In this tech blog, we have verified the predictive accuracy of Spotlight™ compared to previous research.
The previous research used for comparison.
In Li et al., 2022, a machine learning model (DLKcat) was created to predict kcat using enzyme amino acid sequences and compounds as input information. To ensure equality in the comparison, we utilized the DLKcat machine learning model algorithm and reconstructed the model using the same training data as Spotlight™, namely the kcat entries from BRENDA. We compared the predicted kcat values of the mutants by the reconstructed DLKcat and Spotlight and evaluated which values were closer to the actual measured values. For this study, we extracted entries from BRENDA, ensuring that only wild type (WT) and single mutant variants were included. Our focus was to compare the sensitivity for a single mutation between the two models.
Results
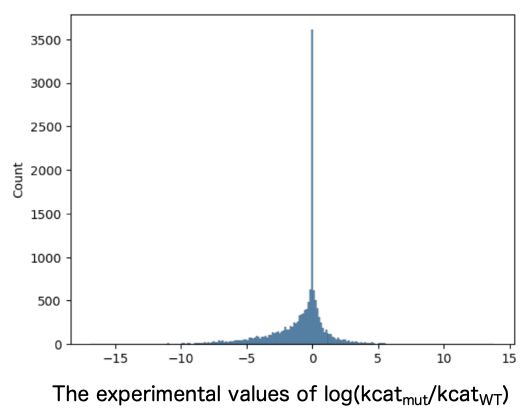
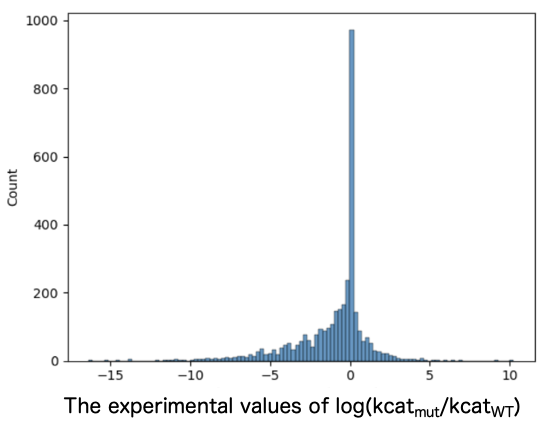
1. Construction of the machine learning model using BRENDA’s kcat (Turnover Number) data.
Entries for variants with reported kcat values were extracted from BRENDA, including entries for the corresponding wild-type (WT) sequences and information about the compounds used to measure kcat. While ensuring no bias toward specific enzyme families, the entries were divided into a 3:1 ratio of training to test data. Training data consisted of 3,969 entries with an increased kcat, 2,985 entries with an unchanged kcat, and 8,296 entries with a decreased kcat (Figure 1). The test data consisted of 792 entries with an increased kcat, 748 entries with an unchanged kcat, and 1,926 entries with a decreased kcat (Figure 2).
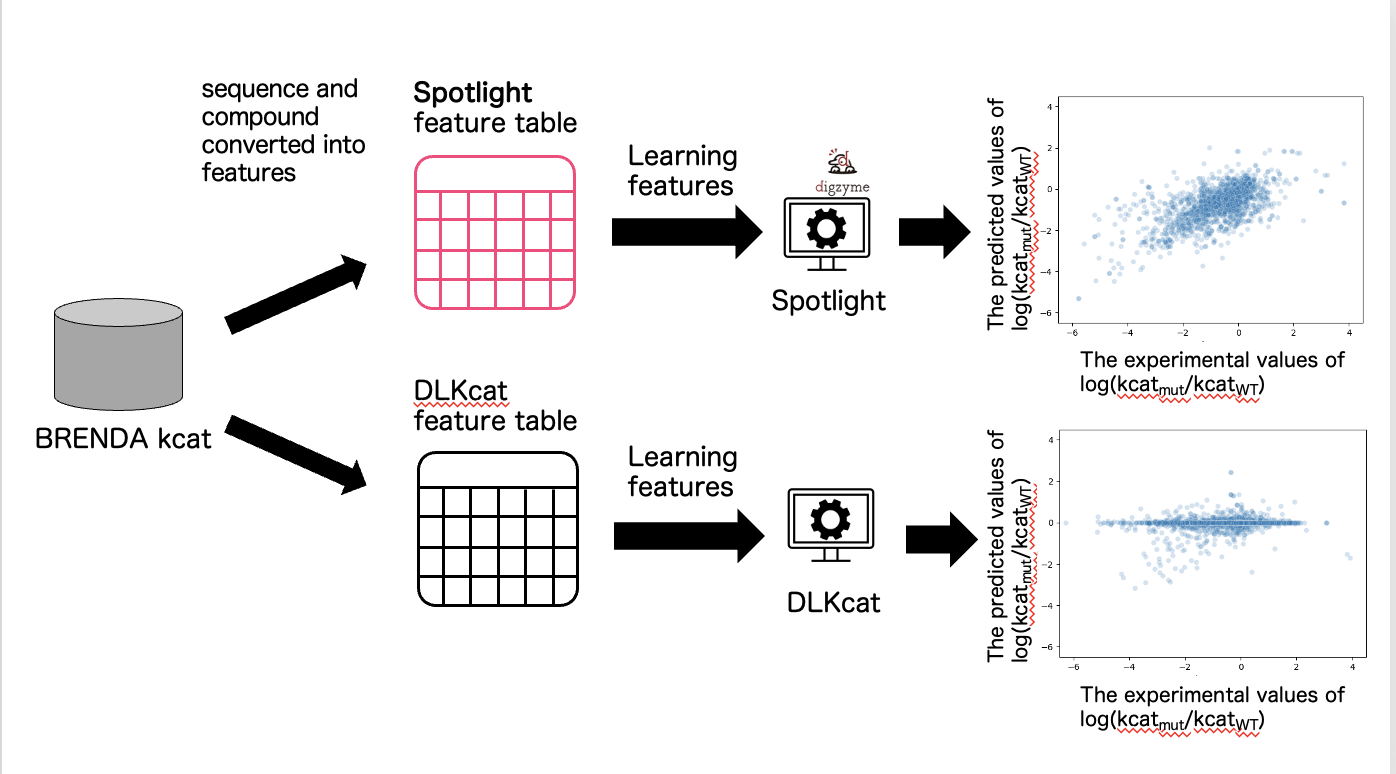
2. Evaluation of mutant/WT ratio of predicted kcat by DLKcat and Spotlight™.
The information from the training data was converted into the format of features required by DLKcat, and a machine learning model was constructed. We converted the training data into the required format of features for Spotlight™ and built a machine learning model (Figure 3).
In the case of DLKcat, the Pearson correlation coefficient between the measured and predicted values of the ratio of the kcat of the mutant to the wild type (WT) kcat was 0.18 (Figure 3). We believe that the reason why the predicted values in DLKcat did not correlate well with the measured values is that DLKcat converts the entire length of the sequence into a vector as a feature, making it difficult for the difference of one amino acid to be reflected in the feature.
In the case of Spotlight™, the Pearson correlation coefficient between the measured and predicted values of the ratio of the kcat of the mutant to the WT kcat was 0.66 (Figure 3). We believe that our Spotlight™ is able to accurately predict the changes caused by single mutations from the WT because it has been devised to reflect the properties of the mutant as a feature.
Conclusion
Our Spotlight™ model was found to more accurately predict changes in activity compared to previous research in cases where only one amino acid mutated.
Acknowledgments
We are grateful for the use of data from the following paper to compare the accuracy of enzyme activity prediction in this study.
Li et al., (2022) Deep learning-based kcat prediction enables improved enzyme-constrained model reconstruction. Nature Catalysis.
